Avogadro’s number is thus the link between the macro world of grams and kilograms, that which we can directly measure, and the micro world of atoms and molecules, about which we can conceive. So moles of Ca = 183.08 ⋅ g 40.078 ⋅ g ⋅ mol−1 = approx. 4.5 ⋅ mol. Number of calcium atoms = 4.5 ⋅ mol ×6.02247 ×1023 ⋅ mol−1 = ??how many calcium atoms
Cells | Free Full-Text | Calcium Channels in the Heart: Disease States and Drugs
You find the molar mass of calcium metal, it is listed as 40.1⋅ g ⋅ mol−1. You should check your copy of the Periodic Table to see if I have got it right. This means that Avogadro’s number of calcium atoms, i.e. 6.022 × 1023 individual calcium atoms have a mass of 40.1 ⋅ g.

Source Image: mdpi.com
Download Image
Step 2/3. 2. Calculate the number of moles of calcium in 179 g: n = m/M = 179 g / 40.08 g/mol = 4.46 mol. Answer. 3. Calculate the number of atoms in 4.46 mol of calcium: One mole of any element contains Avogadro’s number of atoms, which is 6.022 x 10^23.
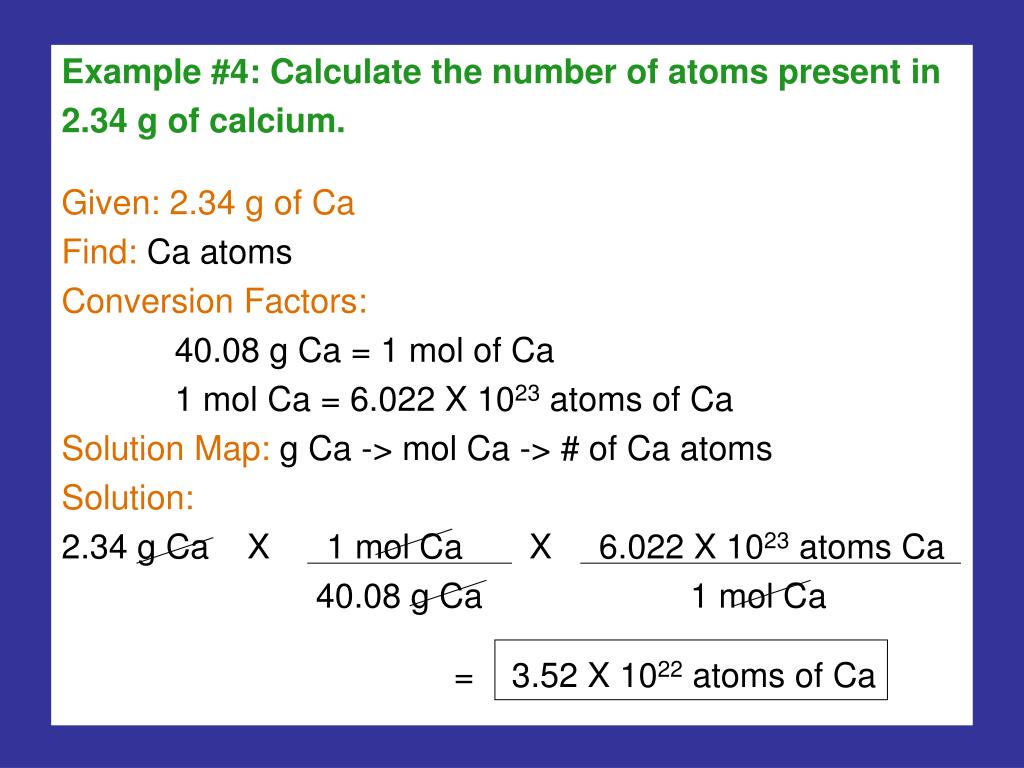
Source Image: slideserve.com
Download Image
Structure−Function Relationship of Calcium Alginate Hydrogels: A Novel Crystal-Forming Engineering | Crystal Growth & Design
1 Answer Nathan L. Jun 7, 2017 2.30 ×1024 atoms Ca Explanation: We’re asked to calculate the number of atoms of Ca in 153 g Ca. What we must first do is convert the given mass of calcium to moles of calcium, using its molar mass (referring to a periodic table, this is 40.08 g mol ): 153 g Ca( 1mol Ca 40.08g Ca) = 3.82 mol Ca

Source Image: pubs.acs.org
Download Image
How Many Atoms Are In 179 G Of Calcium
1 Answer Nathan L. Jun 7, 2017 2.30 ×1024 atoms Ca Explanation: We’re asked to calculate the number of atoms of Ca in 153 g Ca. What we must first do is convert the given mass of calcium to moles of calcium, using its molar mass (referring to a periodic table, this is 40.08 g mol ): 153 g Ca( 1mol Ca 40.08g Ca) = 3.82 mol Ca
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Calcium Ion-Induced Structural Changes in Carboxymethylcellulose Solutions and Their Effects on Adsorption on Cellulose Surfaces | Biomacromolecules
Apr 28, 2022How many atoms are in 179 g of calcium? Updated: 4/28/2022 Wiki User ∙ 10y ago Study now See answer (1) Best Answer Copy 179g/40.078g/mol is 4.46629 moles, x6.022×1023 is 2.6896×1024
Answered: How many moles of calcium atoms do you… | bartleby

Source Image: bartleby.com
Download Image
An Intermetallic CaFe6Ge6 Approach to Unprecedented Ca−Fe−O Electrocatalyst for Efficient Alkaline Oxygen Evolution Reaction – Yang – 2022 – ChemCatChem – Wiley Online Library
Apr 28, 2022How many atoms are in 179 g of calcium? Updated: 4/28/2022 Wiki User ∙ 10y ago Study now See answer (1) Best Answer Copy 179g/40.078g/mol is 4.46629 moles, x6.022×1023 is 2.6896×1024

Source Image: chemistry-europe.onlinelibrary.wiley.com
Download Image
Cells | Free Full-Text | Calcium Channels in the Heart: Disease States and Drugs
Avogadro’s number is thus the link between the macro world of grams and kilograms, that which we can directly measure, and the micro world of atoms and molecules, about which we can conceive. So moles of Ca = 183.08 ⋅ g 40.078 ⋅ g ⋅ mol−1 = approx. 4.5 ⋅ mol. Number of calcium atoms = 4.5 ⋅ mol ×6.02247 ×1023 ⋅ mol−1 = ??how many calcium atoms
Source Image: mdpi.com
Download Image
Structure−Function Relationship of Calcium Alginate Hydrogels: A Novel Crystal-Forming Engineering | Crystal Growth & Design
Step 2/3. 2. Calculate the number of moles of calcium in 179 g: n = m/M = 179 g / 40.08 g/mol = 4.46 mol. Answer. 3. Calculate the number of atoms in 4.46 mol of calcium: One mole of any element contains Avogadro’s number of atoms, which is 6.022 x 10^23.

Source Image: pubs.acs.org
Download Image
atomic mass of calcium is 40.how many atoms are present in 2g of calcium – Brainly.in
About this tutor ›. Avogadro’s number = 6.02×10 23 particles per mol (particles can be atoms, molecules, formula units, etc) atomic mass Ca = 40.08 g / mol. moles Ca = 3456 g x 1 mol / 40.08 g = 86.228 moles. atoms of Ca = 82.228 moles x 6.02×10 23 atoms / mol = 519.1×10 23 atoms = 5.191×10 25 atoms of Ca. ANSWER (a)

Source Image: brainly.in
Download Image
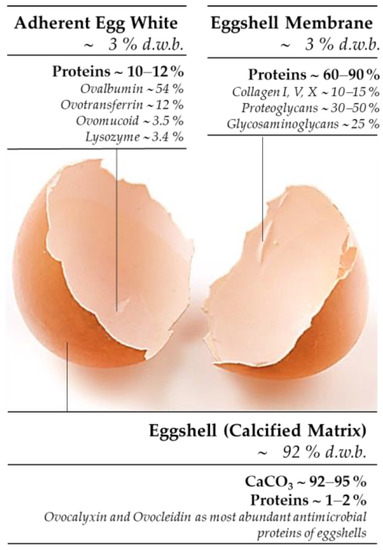
Applied Sciences | Free Full-Text | Eggshell-Waste-Derived Calcium Acetate, Calcium Hydrogen Phosphate and Corresponding Eggshell Membranes
1 Answer Nathan L. Jun 7, 2017 2.30 ×1024 atoms Ca Explanation: We’re asked to calculate the number of atoms of Ca in 153 g Ca. What we must first do is convert the given mass of calcium to moles of calcium, using its molar mass (referring to a periodic table, this is 40.08 g mol ): 153 g Ca( 1mol Ca 40.08g Ca) = 3.82 mol Ca
Source Image: mdpi.com
Download Image
Materials | Free Full-Text | Impact of the Atomic Packing Density on the Properties of Nitrogen-Rich Calcium Silicate Oxynitride Glasses
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.

Source Image: mdpi.com
Download Image
An Intermetallic CaFe6Ge6 Approach to Unprecedented Ca−Fe−O Electrocatalyst for Efficient Alkaline Oxygen Evolution Reaction – Yang – 2022 – ChemCatChem – Wiley Online Library
Materials | Free Full-Text | Impact of the Atomic Packing Density on the Properties of Nitrogen-Rich Calcium Silicate Oxynitride Glasses
You find the molar mass of calcium metal, it is listed as 40.1⋅ g ⋅ mol−1. You should check your copy of the Periodic Table to see if I have got it right. This means that Avogadro’s number of calcium atoms, i.e. 6.022 × 1023 individual calcium atoms have a mass of 40.1 ⋅ g.
Structure−Function Relationship of Calcium Alginate Hydrogels: A Novel Crystal-Forming Engineering | Crystal Growth & Design Applied Sciences | Free Full-Text | Eggshell-Waste-Derived Calcium Acetate, Calcium Hydrogen Phosphate and Corresponding Eggshell Membranes
About this tutor ›. Avogadro’s number = 6.02×10 23 particles per mol (particles can be atoms, molecules, formula units, etc) atomic mass Ca = 40.08 g / mol. moles Ca = 3456 g x 1 mol / 40.08 g = 86.228 moles. atoms of Ca = 82.228 moles x 6.02×10 23 atoms / mol = 519.1×10 23 atoms = 5.191×10 25 atoms of Ca. ANSWER (a)